Wound exudate is produced as a natural and essential part of the healing process. Exudate provides a moist wound environment, enables the diffusion of cells and growth factors and acts as a medium for the migration of tissue-repairing cells across the wound bed. It also helps to supply essential nutrients for cell metabolism and promotes separation of dead or damaged tissue by a process called autolysis (World Union of Wound Healing Societies [WUWHS], 2019).
The aims of exudate management are to:
- Optimise wound bed moisture level as appropriate for the patient and their wound
- Protect the periwound skin
- Manage symptoms and improve patient quality of life (WUWHS, 2019).
What is Suprasorb® Liquacel Pro?
Suprasorb
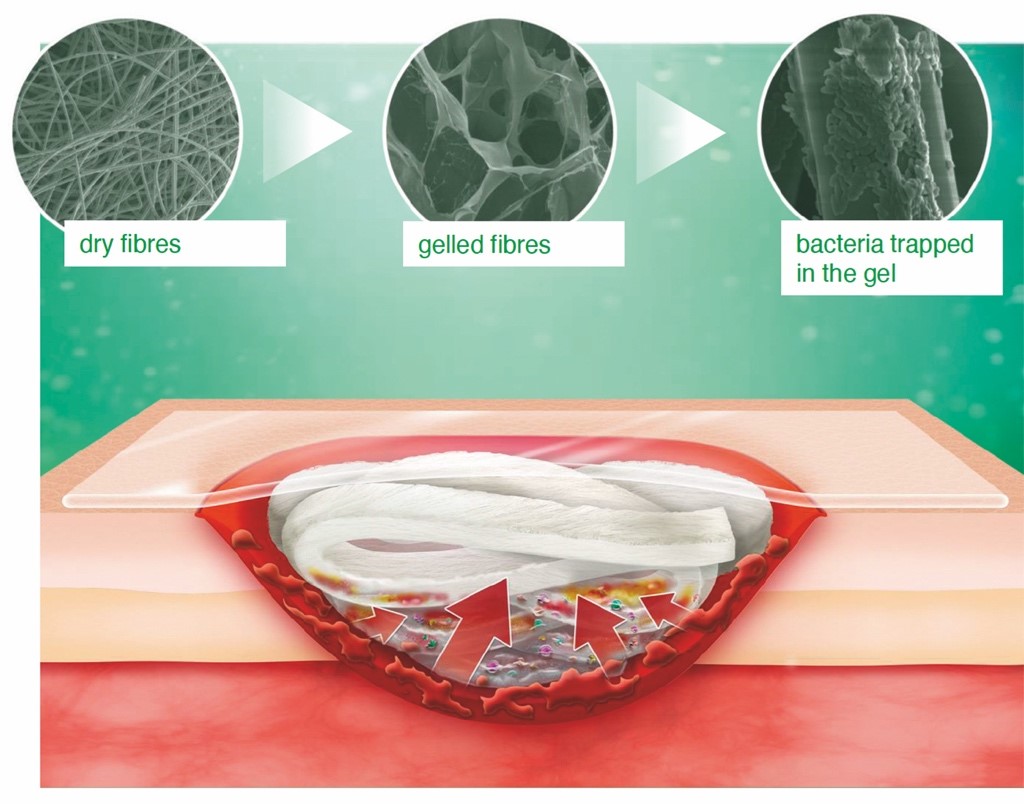
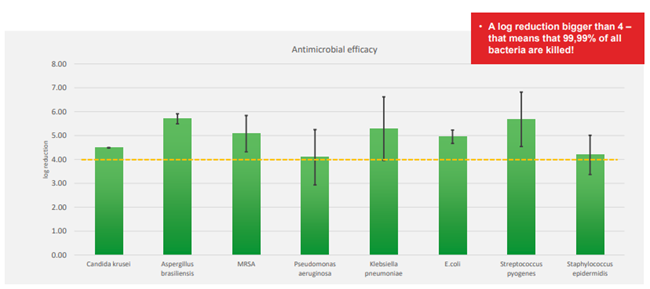
® Liquacel Pro (figure 1) is a soft, absorbent primary wound dressing used to manage exudate. When in contact with wound exudate it forms a gel, which retains its shape and maintains a moist wound environment, supporting wound healing and autolytic debridement. The gel-forming fibres closely conform to the wound bed, leaving no space or gap for bacteria to grow. Exudate is absorbed vertically, protecting the wound edge and the surrounding skin from maceration. Bacteria and cell debris are locked-in to the dressing and removed during dressing changes (reducing the microbial burden). Exudate, cell debris and bacteria are absorbed into the dressing even under compression.
The product is made up of 80% gel forming sodium carboxymethylcellulose fibres (CMC fibres) and 20% strengthening fibres. Suprasorb
® Liquacel Ag is made up of 60% CMC fibres, 40% blend of Lyocell
® and silver fibres (with 1.1% silver nanoparticles).
The cellulose used in production of the Suprasorb
® Liquacel Pro and Suprasorb
® Liquacel Ag CMC fibres and Lyocell
® fibres is derived from wood pulp from sustainable forests , usually eucalyptus trees. During the production process, more than 99% of the solvents used are recovered and reused within a closed chemical loop, with a focus on environmental responsibility.